Lewis Structure S2o3
Data: 3.09.2017 / Rating: 4.8 / Views: 586Gallery of Video:
Gallery of Images:
Lewis Structure S2o3
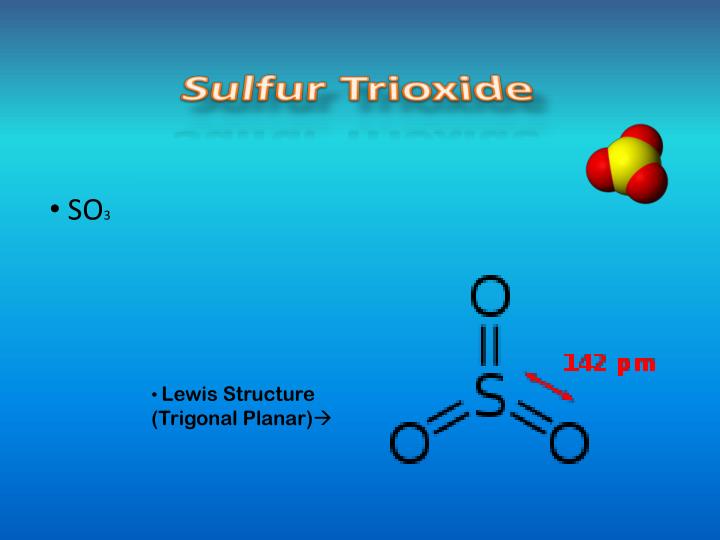
Transcript: Hi, this is Dr. Let's do the SO3 2 Lewis structure. For the SO3 2 compound, we have 26 total valence electrons, and that includes these two electrons. In the Lewis structure of the iodate ion, IO3, that satisfies the octet rule, the formal charge on the central iodine atom is? Draw a Lewis structure for each of the following molecules and determine its. Some are stable molecules and some including S2O2 and S2O3 decompose when they are heated. Draw lewis structure for these two compounds showing all resonance forms. Mar 13, 2015Lewis Structure of Sulfate and Thiosulfate. The thiosulfate anion resembles sulfate except that one of the oxygen atoms has been exchanged with a sulfur atom. Dec 05, 2009What is the structure of S4O6 (2)? The actual question was to find the oxidation state of each sulphur atom, but I did not know its structure. The hybridization of the atoms in this idealized Lewis structure is given in the table Return to Molecular Structure Page. Sep 07, 2006How would I drawa a Lewis Dot Structure for [S2O3 2 Sorry, can't do the subscript and superscript here. Answer to Draw Lewis structures for all resonance forms of S2O3. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure. Oct 13, 2008(had this doubt while trying to draw the structure of S2O3(2)) 3) how to claculate formal charge and oxidation number in general Drawing Lewis structure The structure of a thiosulfate anion is a slightly distorted tetrahedral shape in which the central atom is sulfur with three surrounding oxygen atoms and a second. Disulfur monoxide or sulfur suboxide is an inorganic compound with formula S 2 O. It is one of the lower sulfur oxides. It is a colourless gas and condenses to give a. Mar 13, 2015This video shows you how to draw the lewis structure for the thiosulfate ion S2O3 2. Structure, properties, spectra, suppliers and links for: thiosulfate, thiosulphate, S2O3(2). Resonance: Drawing Resonance Structures The Lewis structure for this ion has a carbonoxygen double bond, and two carbonoxygen single bonds. The structure of the thiosulfate anion. The prefix thioindicates that the thiosulfate ion is a sulfate ion with one oxygen replaced by sulfur. You need to consider the Lewis structure of this ion. According to the structure, two of the S atoms (color What is the oxidation number of sulfur in S4O62. Sometimes you will have multiple resonance structures which do not contribute equally to the final we calculate the formal charge on each atom in a Lewis structure. Sep 10, 2014How to draw the Lewis Structure of SO3 (sulfur trioxide) with explanation Sulfur is an exception to the octet rule it can handle up to 12 electrons. S2O2: Draw Lewis structure showing all resonance forms.
Related Images:
- Engineering Electromagnetic Fields And Waves
- Ole Normale Supeureepub
- The Elder Scrolls V Skyrim Special EditionCODEX
- 17519 Nvidia Driver Windows 7zip
- Driver download pc game ultima
- Carmageddon 1 no cd crack
- John Deere Manual For L110
- Spartito profondo rosso goblin pianoforte
- Shrek 3
- Forgotten Civilization
- The Makarov Pistol Soviet Union
- Ti King Album Zip
- Fundamentals Of Statistics 1st Edition
- Harry Potter a vezen z Azkabanu Harry Potter 3
- Motiv hair mp3
- Field Study 2 Episode 19 Answers
- Materi sosiologi pendidikan pdf
- How To Change Manual Transmission Fluid Mazda 6
- Les Musees Sont Des Mondes
- Pensieri e parole una vita a metatorrent
- Le origini Dragoneropdf
- Mifare offline cracker gui windows javascript
- 3rd grade science on ecosystemspdf
- Soil tester takemura dm 5
- Lucky Luke Pdf
- 30 Days To A More Powerful Vocabulary
- Gli occhi verdipdf
- Bahishti zewar pdf english
- Charlaine Harris Night Shift
- Ms word practical exercises with solutions
- HP 550 Acpi Driverzip
- Messianic Torah Commentary Gary Gardner Ebook
- Employment insurance sickness benefits medical form
- Signature Ep01 FR
- Death and the Maiden
- Functional requirements examples software engineering
- Explore Learning Mystery Powder Analysis Answers Gizmo
- Red Faction I Full Game not ripped
- Chocolate Decorations
- Manualse Ape Piaggio Tm 703
- Ngs Web camera Driver freezip
- Como descargar tcx converter download
- Mercury 650 65 Hpoutboard Repair Manuals
- I tesori dellisola di Pianosa nel mar Tirrenoepub
- 2008 Range Rover Repair Manuals
- General Chemistry 4th Edition
- Mdaemon server free
- Mapinfo professional
- Farmacologia Generale E Molecolare Utet Pdf
- Massey Ferguson 245 Orchard Tractor For Sale
- Microsoft outlook 2010 training manuals
- Kivy Cookbook Solis Hugo
- Mdaemon Mail Server Full Crack
- Bramadiviverepdf
- BBPR La torre Velascadoc
- Atlas de anatomia werner platzer pdf
- I giardini di Inannaepub
- Ghjibdrf Android Xthtp Rjvgm
- Panasonic Tbm2ax13001 Manualpdf
- Hider 221dmg OS X
- INTERICAD T6 FULL CRACKEDISO
- Flow chart for fry chickenpdf
- 1020 Case Ih Grain Head Manual
- Backstreet Boys Unbreakable By Backstreet Boys
- Age Of Empires 1 No Cd Crack Free
- Lim seulong girlfriend activation
- Que mosca pongo guia de bolsillo
- Yugioh joey the passion nocd crack fs2004
- Aexio xeus pro
- Application letter for work immersion
- Sirith maldama PDF
- Realidades La Conversacion Completa Answers